Introduction
Scope of the Institute for Auditory Neuroscience
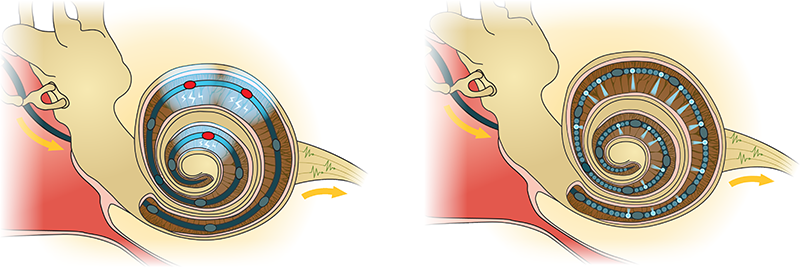
The Institute for Auditory Neuroscience targets the molecular anatomy, physiology, pathophysiology and restoration of synaptic information processing in the auditory pathway. We aim to elucidate the specialized molecular and cellular mechanisms that enable information processing with rates of hundreds per second over hours with submillisecond temporal precision. We combine complementary approaches to dissect the structure and function of hair cell ribbon synapses in the cochlea and of large calyceal central auditory synapses from the molecular level to systems function.
of the brainstem.
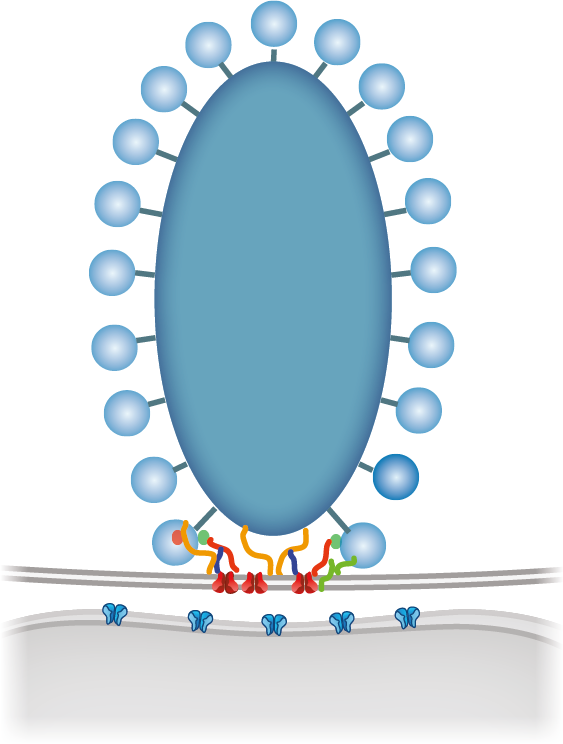
by scaffolding proteins such as bassoon, RIM, and ribeye.
Transmission at the large calyceal synapses of the central pathway are similarly specialized in synaptic transmission, at rates of hundreds of Hertz but employ strategies that differ greatly from those of the hair cell synapse. Here, hundred(s) of active zones with conventional molecular anatomy operate in parallel in the large presynaptic terminals that wrap around the postsynaptic soma to safely excite the principal cells with great temporal precision. We argue that even subtle deficits in the regulation of vesicle dynamics and neurotransmitter release will be revealed at synapses with such high functional demands.
Structure of the Institute for Auditory Neuroscience
The Institute for Auditory Neuroscience has its home at the University Medical Center Göttingen, where it closely interacts with groups of the Dept. of Otolaryngology within the joint InnerEarLab. The individual groups combine their complementary expertise and perspectives to jointly tackle longstanding neuroscience questions. The Institute for Auditory Neuroscience also holds strong ties to partner institutions at the Göttingen Campus, which has led to the establishment of satellite groups at the Max-Planck-Institute for Biophysical Chemistry (Synaptic Nanophysiology group, link), the German Primate Center (Auditory Neuroscience group, link) and the Max-Planck-Institute for Experimental Medicine (Auditory Neuroscience group, link). The Institute for Auditory Neuroscience is part of several research networks at the Göttingen Campus. Most notably it forms a core of the collaborative sensory research center 889 (SFB, link) and of the Graduate program on Sensory and Motor Neuroscience (link).
Molecular anatomy, physiology and pathology of sound encoding at hair cell ribbon synapses
- Ca2+ nanodomain control of exocytosis.
We hypothesize based on biophysical experiments and modeling that the Ca2+ at the Ca2+ sensor of a given release ready vesicle is dominated by the contribution of the nearest Ca2+ channel (Ca2+ nanodomain control of exocytosis). We argue that this enables synaptic sound encoding to proceed with high temporal precision also for weak sounds, because once a Ca2+ channels opens this leads to a saturating Ca2+ at the Ca2+ sensor and a quite invariant kinetics of vesicle fusion. - Uniquantal release with regulation by a dynamic fusion pore.
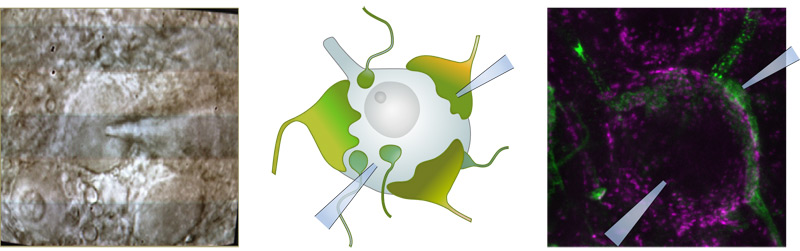
The postsynaptic spiral ganglion neuron receives input just from one active zone and with a large number of ionotropic glutamate receptors represents a highly sensitive readout of single active zone function. Recording from the postsynaptic boutons reveals large heterogeneity in amplitude and shape for the excitatory postsynaptic currents (EPSC). We hypothesize based on biophysical experiments and modeling that release at this synapse is primarily uniquantal and that regulation of release by the vesicular fusion pore contributes to EPSC heterogeneity. We propose that due to efficient postsynaptic glutamate detection at this synapse single vesicle release is turned into a postsynaptic spike. - Active zone clearance is of great relevance for efficient vesicle replenishment.
Active zones of inner hair cells release hundreds of vesicles per second. This implies that the approximately one dozen release sites must reload new releasable vesicles at tens of Hz. How the previously fused exocytic material is moved out of the release site is not well understood but likely facilitated by molecular interactions between exo- and endocytic proteins. - Hair cell ribbon synapse bears a highly specialized molecular machinery.
We hypothesize that deviates from the make-up of small CNS synapses. Each of the 5-20 active zones drives spiking in one spiral ganglion neuron in the absence and presence of acoustic stimulation. High and sustained rates of synaptic transmission require very efficient means of vesicle cycling. It becomes evident that interfering with the hair cell vesicle cycle results in auditory dysfunction, be it vesicle docking, priming, fusion or endocytic membrane retrieval. The synapses of one hair cell differ from each other in structure and function, most probably in order to cover the broad range of sound pressures with the limited dynamic range of encoding at each individual synapse. - Hair cells employ heterogeneity of their synapses in order to decompose auditory information in diverse neural channels.
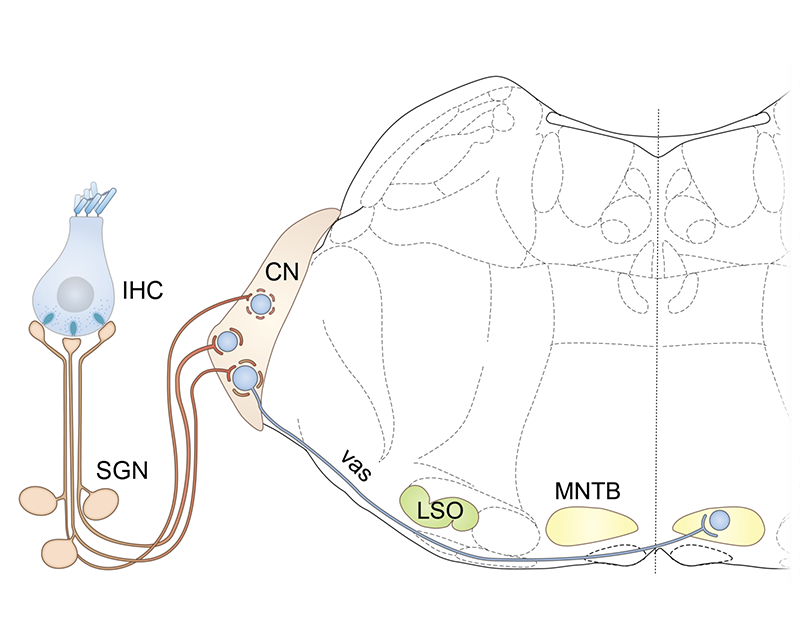
The endbulb and calyx of Held synapses are large, craw-like or calyceal presynaptic terminal with more than one hundred small “conventional” active zones. The endbulb of Held Synapse acts as a reliable and temporally precise relay synapse. 3-5 endbulbs likely arising from different spiral ganglion neurons collectively drive the bushy cell, which acts as a coincidence detector.
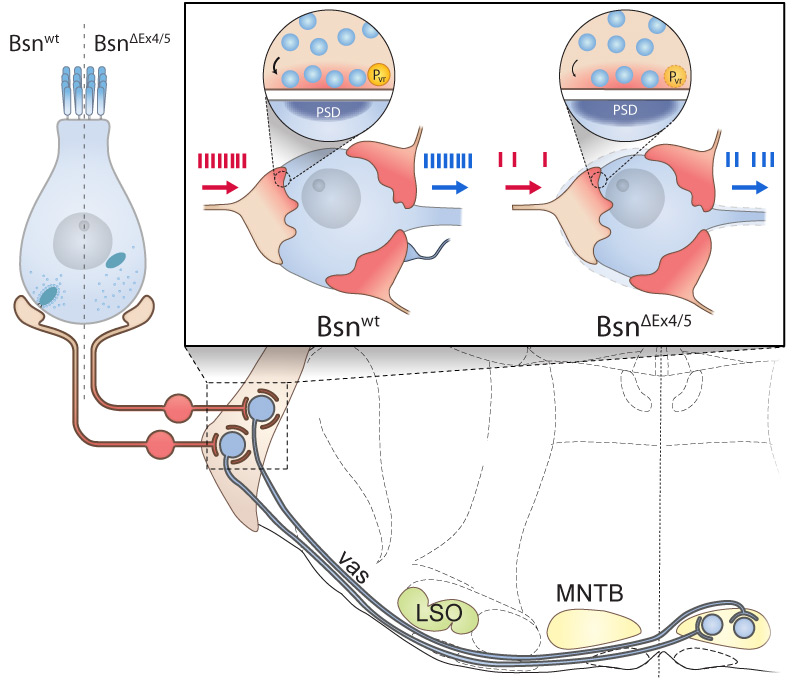
such as in mutants lacking the functional bassoon scaffold protein.
The synapses of one hair cell differ from each other in structure and function, most probably in order to cover the broad range of sound pressures with the limited dynamic range of encoding at each individual synapse. The endbulb and calyx of Held synapses are large, craw-like or calyceal presynaptic terminal with more than one hundred small “conventional” active zones. The endbulb of Held Synapse acts as a reliable and temporally precise relay synapse. 3-5 endbulbs likely arising from different spiral ganglion neurons collectively drive the bushy cell, which acts as a coincidence detector.
Fast, precise and reliable transmission at synapses of the central auditory system
The endbulb and calyx of Held synapses are large, craw-like or calyceal presynaptic terminal with more than one hundred small “conventional” active zones. The endbulb of Held Synapse acts as a reliable and temporally precise relay synapse. 3-5 endbulbs likely arising from different spiral ganglion neurons collectively drive the bushy cell, which acts as a coincidence detector improving temporal precision of firing. The endbulb of Held Synapse is a reliable and temporally precise relay synapse.
Restoration of sound encoding in the cochlea
When hearing fails due to genetic or exogenous causes hearing aids or cochlear implants currently provide the only means of hearing restoration. As well documented for Leber’s Amaurosis of the eye, viral gene therapy approaches can greatly benefit people suffering from sensory deficits. We are currently employing viral gene transfer for two synaptopathy genes in mice, which may offer an entry point for devising causal treatment of select hereditary deafness.
We also employ virus-mediated expression of the light-gated ion channel channelrhodopsin in cochlear spiral ganglion neurons. Combined with innovative optical stimulation architecture this, we hope, will help to improve the state of the art cochlear implant. Towards this goal we, in collaboration with the Freiburg groups of Schwarz and Ruther develop prototypic devises and characterize optogenetic stimulation of the auditory nerve from the cochlea to auditory cortex and behavior.