Introduction to the Institute for Auditory Neuroscience
The Institute for Auditory Neuroscience targets the molecular anatomy, physiology,
pathophysiology and restoration of synaptic information processing in the auditory
pathway.
We aim to elucidate the specialized molecular and cellular mechanisms that enable
information processing with at rates of hundreds per second over hours with
submillisecond
temporal precision. We combine complementary approaches to dissect the structure and
function of hair cell ribbon synapses in the cochlea and of large calyceal central
auditory
synapses from the molecular level to systems function.
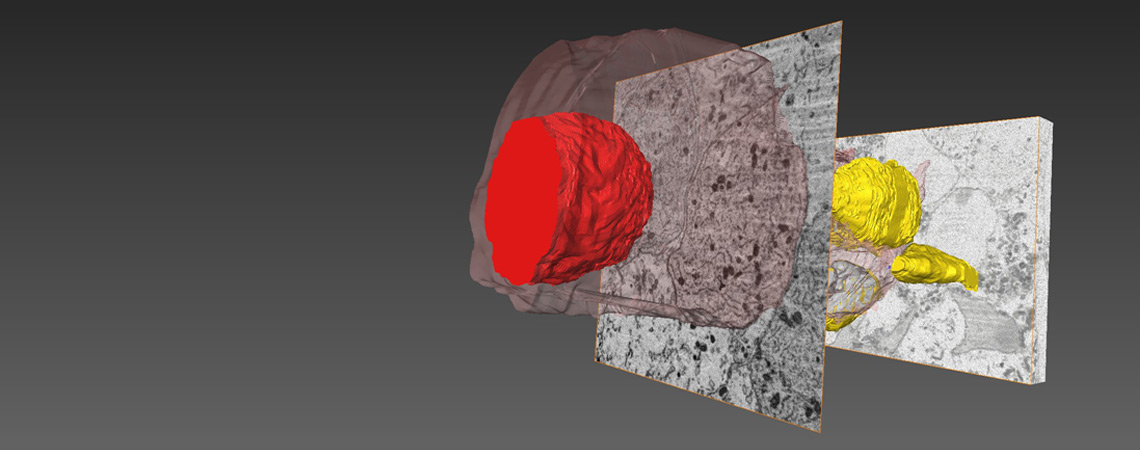
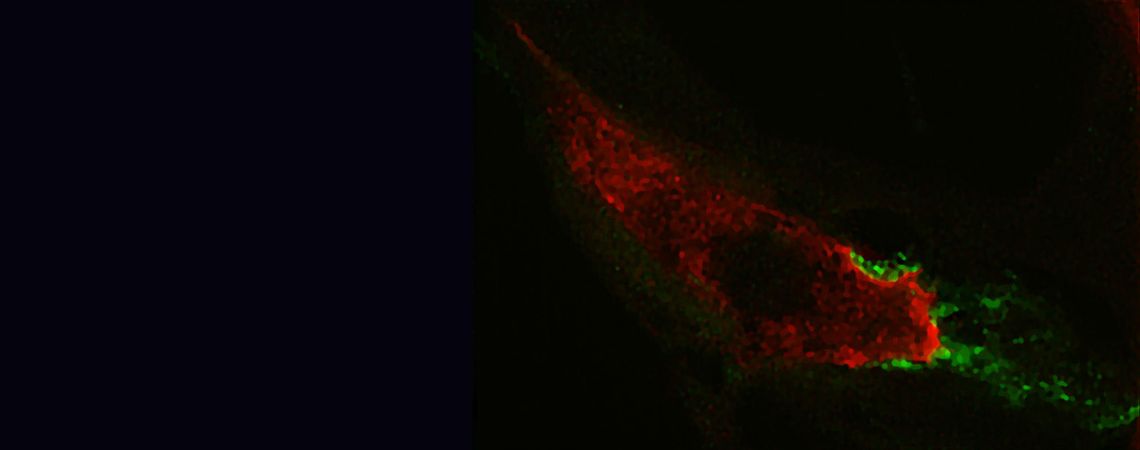
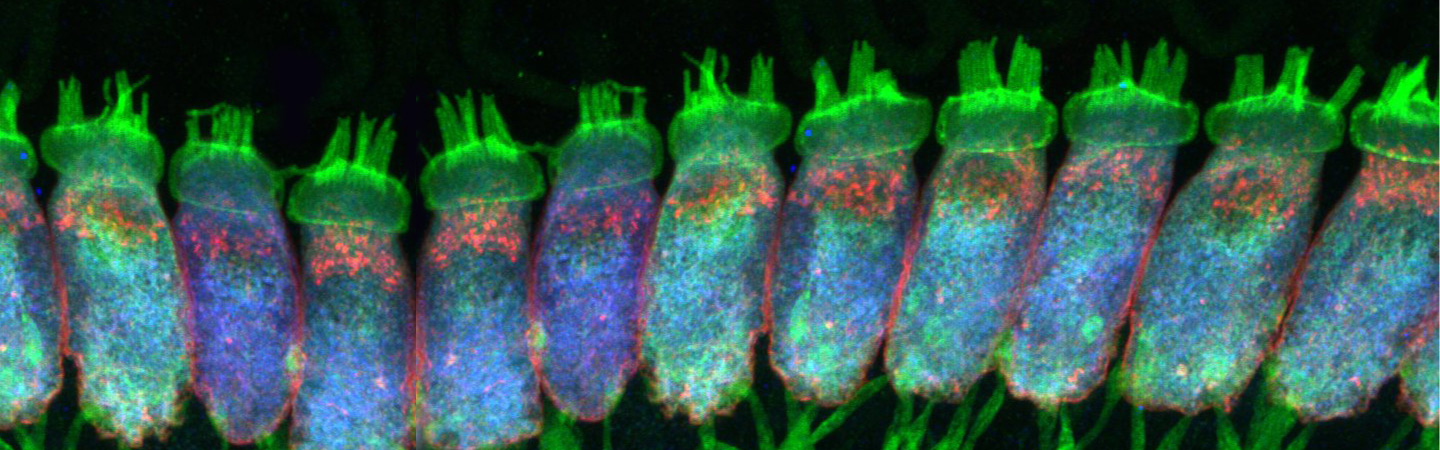
The hair cell synapse features a single and large ribbon-type active zone.
When
hair cells transduce the mechanical stimulus into an electrical signal, voltage-gated
Ca2+ channels open and the ensuing Ca2+ influx triggers exocytosis
of
glutamate filled vesicles at the ribbon synapses. Our work on the hair cell ribbon
synapse
addresses fundamental questions such as “How is the high temporal precision of the
auditory
code brought about by a chain of stochastic events at the hair cell synapse?” and “How
can a
nanoscale membrane-domain turnover hundreds of vesicles without jamming and loss of
molecular and structural identity? Evolved for speed, precision and inexhaustibility the
synapse seems to employ intriguing synaptic mechanisms of Ca2+
channel-release
site coupling, exocytosis, clearance from exocytosed material from the active zone and
endocytic vesicle recycling as well as glutamate detection and action potential
generation.
Work over the past two decades has elucidated an unconventional molecular composition
that
likely explains the existence of genetic defects of the hair cell synapse (auditory
synaptopathy) in humans, which leads to hearing impairment often in the absence of other
symptoms. We work on developing gene therapeutic approaches to restore function in
select
cases of hereditary synaptopathy. For cases where such restoration of cochlear function
is
not emanable we aim improve the performance of cochlear implants by harnessing the
potential
of optogenetics for spatially more confined stimulation of the spiral ganglion. Finally,
we
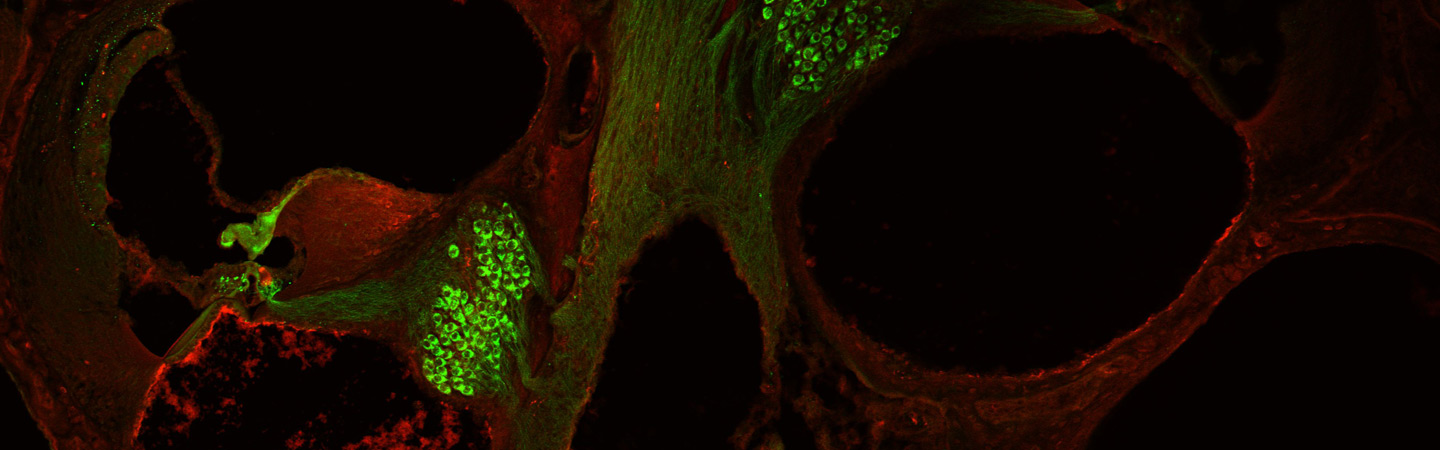
have obtained evidence for major synaptic heterogeneity even within a given hair cell.
We
hypothesize that such synaptic heterogeneity enables the inner hair cell to decompose
auditory information into functionally distinct neuronal channels to the brain.